Nanostructured electrodes improve sensor sensitivity with faster electron transfer
By Andy Tay
Electrochemical sensors make use of electrodes as a transducer element to convert chemical signals attributing to a target analyte into electrical signals for detection. They are developed as part of integrated circuits for biomolecular sensing through low cost, miniaturized device. One of the key considerations for using an electrochemical sensor in biomedical application is its sensitivity as many clinical biomarkers in the body like proteins and lipids are present in nano- to pico-molar levels mixed with potential interfering biomolecules. Electrochemical sensors can also help to monitor the concentrations of drugs such as doxorubicin used in cancer therapy in biofluids. This is useful to maximize therapeutic dose and efficacy while minimizing adverse response from high drug concentration.
However, when electrochemical biosensors are miniaturized, the signal to noise ratio regresses which adversely affect their sensitivities. Nanostructured electrodes have been shown to demonstrate superior sensing properties including better signal levels and faster diffusion of redox species to achieve high higher limit of detection for hybridization-based nucleic detection compared to their planar counterparts.
In a recent paper published in Advanced Science, a team of researchers led by Professor Hyongsok Tom Soh, Dr. Kaiyu Fu, Dr. Ji-Won Seo and Vladimir Kesler and supported by Professor Boris Murmann in the Department of Electrical Engineering at Stanford University found a new mechanism that enhanced the sensitivity of aptamer-based electrochemical sensors (Fu et al., 2021). Specifically, nanoporous electrode structures decreased Debye volume and reduced the effect of charge screening within nanopores to accelerate electron transfer between redox-tagged aptamer that mediates target recognition, and the surface of gold electrode.
“An obvious advantage of the nanostructured electrode compared to the flat electrode is enhancing electrode surface area, which leads to a larger electrochemical signal and better signal-to-noise ratio. Over the years, several nanostructured electrodes have been proposed, including a pioneering work by Soleymani et al. in 2009 which described an elegant way to tune the nanostructures for nucleic acid detection over a wide dynamic range. However, one major hurdle for long term in vivo biosensing is device stability against the biofouling and biodegradation environment inside animals. The nanoporous electrode reported in this work, for the first time, highlights size-tunable concave nanostructures, where the biosensor’s sensitivity and antifouling capability are greatly improved. As a result, we can achieve nearly 24-fold greater signal output and a 4-fold lower detection limit,” says Fu.
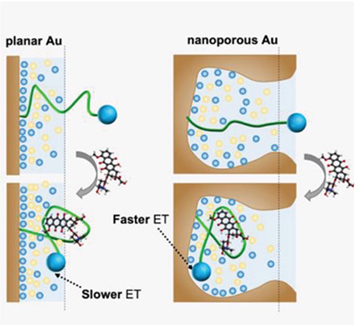
Nanoporous electrode performs better than planar electrode
To verify literature findings that nanoporous electrode is more sensitive than planar electrode, Fu et al. immobilized doxorubicin aptamer onto planar and nanoporous electrodes via gold-thiol interaction. Doxorubicin aptamer was coupled to a methylene blue redox reporter. Without the target analyte, the aptamer is unfolded and there is limited electron transfer between reporter and electrode. However, target binding causes aptamer to adopt a folded conformation, bringing reporter tag into closer proximity to electrode surface to increase electron transfer to generate signals.
The team also decided to make use of square wave voltammetry to measure the performance of their sensors as it provides better sensitivity and lower background than other characterization methods. This technique works by applying a series of small voltage steps with positive and negative phases to create electric fields in the electrochemical cell to oxidize (positive phase) and reduce (negative phase) the methylene blue tagged to aptamer, to generate a net current. This method was found to generate a higher signal to noise ratio for easier quantification of doxorubicin levels.
Fu and co-workers next fabricated nanoporous electrodes using gold/silver alloys and adjusted the pore sizes in these electrodes. Reduced pore size was found to improve signals where the smallest pore size of 9.3 nm gave a signal gain of 194% compared to 32% for planar electrode at saturating concentration of doxorubicin.
To further confirm that the performance of nanoporous electrode is better than planar electrode, the authors also tested different conditions such as concentration of aptamer molecules. Across all conditions, nanoporous electrode was found to provide better signal gain (3 folds more), signal level (24 folds better) and limit of detection (reduction of four folds) than planar electrode. The team attributed the superior performance of nanoporous electrode to larger surface area for aptamer immobilization which enhanced magnitude and reduce variance of measured signals.
Novel mechanism for signal enhancement
Fu and co-workers hypothesized that the nanoporosity of their electrodes could affect the kinetics of electron transfer between methylene blue redox reporter and electrode by weakening charge screening. This is because high density of nanoscale features is known to reduce charge screening at the electric double layer to accelerate faradaic electron transfer.
First, making use of 2D simulation study, the authors found that nanoporous gold surfaces could diminish the effects of charge screening relative to a planar surface. Next, the authors used square wave voltammetry with samples of different ionic strengths (to affect Debye length in electric double layer) and doxorubicin concentrations. They found that lower ionic strength extended the electric double layer (and Debye length), resulting in higher signal gains and confirming that electron transfer can affect electrode sensitivity. Finally, they made use of chronoamperograms and found that in nanoporous electrode, there was faster faradaic reactions between the redox reporter and electrode, supporting that weakened charge screening in nanoporous electrode accelerated electron transfer.
“Electrochemical aptamer biosensors serve as a promising reagent-free detection platform that can be readily coupled with electronic devices. The strategy described in this work can be applied to virtually any electrochemical aptamer, enabling sensitive detection of small molecules, proteins, and other targets. So far, there is an urgent need to develop a powerful and versatile biosensing platform to track and monitor biomolecules with high sensitivity and specificity. So far, one limitation is the relatively broad nanopore size distribution because of the metal dealloying fabrication method used in this study. A better size-exclusion effect with a narrow cutoff would further prevent the non-specific protein absorption and enzymatic digestion of the aptamer surface inside the nanopore,” says Fu.
Fu adds that the nanoporous electrochemical biosensor is applicable to a wide range of biomolecules in diagnostic and health monitoring applications. As a proof of concept, they had a follow-up study published early this year that utilized this biosensor for real-time monitoring of drug pharmacokinetics within tumor tissue in live animals (Seo et al., 2022).
Overall, this paper discovered a new mechanism to engineer electron transfer for improving the performance of electrochemical biosensors. This could make electrochemical biosensors even more powerful to detect and quantify a wide range of biomolecules for diagnostics and health monitoring applications.




Fu, K., Seo, J.-W., Kesler, V., Maganzini, N., Wilson, B. D., Eisenstein, M., Murmann, B., & Soh, H. T. (2021). Accelerated Electron Transfer in Nanostructured Electrodes Improves the Sensitivity of Electrochemical Biosensors. Advanced Science, 8(23).
Seo, J.-W., Fu, K., Correa, S., Eisenstein, M., Appel, E. A., & Soh, H. T. (2022). Real-time monitoring of drug pharmacokinetics within tumor tissue in live animals. In Sci. Adv (Vol. 8). https://www.science.org
The eWEAR-TCCI awards for science writing is a project commissioned by the Wearable Electronics Initiative (eWEAR) at Stanford University and made possible by funding through eWEAR industrial affiliates program member Shanda Group and the Tianqiao and Chrissy Chen Institute (TCCI®).